VIDEO LECTURE ON BASICS OF THERMODYNAMIC
Contents
Definition of thermodynamics
Basic terms used
Laws of thermodynamics
System, Boundary and Surroundings
Equilibrium
Intensive and Extensive properties
State, Process, Cycle
Point and path functions
Definition
- Thermodynamics is the branch of science deals with relationship between heat and work.
- It deals with transfer of energy within the system and across the boundary of the system.
Basics Terms Used
Mass
Weight
Volume
Density
Specific weight
Specific gravity
Specific volume
Pressure
Temperature
Heat
Work
Power
Energy
Mass
- Mass is the amount of matter contained in an object, and does not depends on gravity.
- Unit of mass is gram, kilogram.
- W=mg
- It is denoted as 'm'
Weight
- The amount of mass depending upon gravity is called weight.
- It is the product of mass and acceleration gravity.
- W=mg.
- Unit is Kgm/sec2 (or) Newton.
- It is denoted as'W'
Volume
- Space occupied by the mass.
- Ratio of mass to density
- It is denoted as a term 'V'
Density
- It is defined by amount of mass per unit volume
- Unit of density is Kg/m3.
It is denoted as a term 'ρ'
Specific weight
- Also called as weight density
- It is defined by ratio of weight to volume
- Unit is N/m3.
- It is denoted as 'w'
Specific gravity
- Also called as relative density
- It is defined by ratio of density of given substance to density of standard substance.
- It has no unit.
- Density of standard liquid (ρw) = 1000 kg/m3.
- It is denoted as a 'S'
Specific volume
- Volume occupied by unit mass is called as specific volume.
- It is the reciprocal of density.
- Unit is m3/Kg.
- It is denoted as 'v'
Pressure
- It is defined as the force over area.
- Units of pressure are N/m2 ,bar, pascal
- Absolute zero pressure is called as perfect vacuum
- Below the atmospheric pressure is called as vacuum pressure.
- If the pressure is measured by means of mechanical gauges that is called as gauge pressure.
- It is denoted as 'P'
Relationship between various pressures
Pressure measuring devices
- Barometer
- Piezo meter
- Manometer
- U tube manometer
- Differtial U tube manometer
- Inverted U tube manometer
- Micromanometers
Pressure gauges
- Bourdon tube pressure gauge
- Dead weight pressure gauge
- Diaphragm type pressure gauge
Temperature
- It is defined as degree of hotness or coldness.
- It is denoted as 'T'
- This is the amount of heat present in a system.
- Unit is degree Celsius (or) degree Fahrenheit (or) Kelvin.
- Four temperature scales available
- Celsius or centigrade temperature scale
- Absolute temperature scale (or) Kelvin temp. scale
- Rankine temperature scale
- Fahrenheit temperature scale
Relationship between kelvin and degree Celsius
- T = t + 273
- t-Temperature reading in Celsius scale
- T-Temperature in kelvin scale
- Freezing point of water is 00C (or) 273K
- Boiling point of water is 1000C (or) 373 K
STP and NTP conditions
Standard Temperature and Pressure
T= 15°C , P=Patm
Normal temperature and pressure
T=0°C , P= Patm
Heat
- Heat is a form of energy. It can be transferred from one body to another due to the difference of temperature.
- Heat is defined as the energy transferred, without transfer of mass, across the boundary of a system because of a temperature difference between the system and the surroundings.
- It is expressed in Joule (J). It is usually represented by ‘Q’.
Sign convention in heat
- Heat received by the system = +Q
- Heat lost or heat rejection by the system = -Q
Q = m C (T2-T1)
Specific heat capacity
It is defined as the amount of heat required to raise or lower the temperature of a unit mass of any substance through one degree. Its unit is kJ/kgK. It is denoted by ‘C’.
Specific heat capacity at constant volume process
It is defined as the amount of heat required to raise or lower the temperature of a unit mass of any substance through one degree when the volume remains constant. Its unit is kJ/kgK. It is denoted by ‘Cv’.
Q = m Cv dT
Where
Q = Heat transferred (kJ)
m = Mass of the gas (kg)
Cv= Specific heat capacity at constant volume (kJ/kgK)
dT =(T2-T1) = Temperature difference (K)
Specific heat capacity at constant pressure process
It is defined as the amount of heat required to raise or lower the temperature of a unit mass of any substance through one degree when the pressure remains constant. Its unit is kJ/kgK. It is denoted by ‘Cp’.
Q = m Cp dT
Where,
Q = Heat transferred (kJ)
m = Mass of the gas (kg)
Cp= Specific heat capacity at constant pressure (kJ/kgK)
dT =(T2-T1) = Temperature difference (K)
Why Cp always greater than Cv?
Work done = P dV
At V=C; Work done = 0
At P=C; Work done = P(V2-V1)
“When the gas is heated up at constant pressure volume will be increased so there is a work done, but incase of constant volume process there is not work done. So for all gases Cp always higher than that of Cv”
Constant values
- Cp– Cv = R
- Cp value of air = 1.005 kJ/kgK
- Cv value of air = 0.718 kJ/kgK
- R (Gas Constant) = 0.287 kJ/kgK
- Ƴ = 1.4
Work
According to mechanics, Work will be done when the point of application of force moves in the direction of force. Its unit is Nm or Joules. It is denoted by ‘W’.
Work done (WD) = Force (F) x Distance moved (d)
According to thermodynamics work done is defined as the energy transferred across the boundary of a system because of an intensive property difference other than temperature exists between system and surroundings.
Work done (WD) = p dV (dV= change in volume)
Unit is Nm or Joule
1Nm = 1 Joule
Sign convention in work
Work done by the system = +W (Output)
Example : Expansion process - Turbine
Work done on the system = -W (Input)
Example : Compression process - Compressor
Power
- Rate of doing work is called as power.
- Unit is W and KW.
- 1Ws=3600J
- It is usually represented as 'P'
Energy
- Capacity to do the work is called as power.
- Unit is J and KJ.
- Stored energy and transit energy are the types of energy.
- Potential energy, Kinetic energy, Internal energy, Flow energy are stored energy.
- Heat energy and work done are transit energy
- Usually denoted as 'E'
Energy formulae
Potential energy = mgh
Kinetic energy = 1/2 mv2
Internal energy = mCvt
Flow energy = PV
Heat energy = mCdT
Work done = PdV
Law of conservation of energy
It states that, “The energy can neither be created nor destroyed, though it can be transformed from one form to any other form, in which the energy can exist”.
Laws of thermodynamics
Zeroth law of thermodynamics
This law states that “when two bodies are separately in thermal equilibrium with a third body, then they are in thermal equilibrium with each other.
Thermometer works under this law.
First law of thermodynamics
When a system undergoes a cyclic process, the algebraic sum of the work transfer is proportional to the algebraic sum of the heat transfer.
For isolated system dQ=0 and W=0
Δ U = Q – W
Second law of thermodynamics
Second law of thermodynamics --- Kelvin plank statement
It is impossible to construct a heat engine working on cyclic process, whose purpose is to convert all the heat energy supplied to it into work.
Second law of thermodynamics --- Clausius statement
It is impossible to construct a system working on a cyclic process whose purpose is to transfer heat from a colder body to a hotter body without the aid of external work
Thermodynamic systems
The thermodynamic system (simply known as system) may be defined as a definite area or a space where some thermodynamic process is taking place.
Types of thermodynamic systems
Open system (or) flow system (or) control volume system
In an open system or flow system or control volume system, both energy and mass (heat and work) cross the boundary of the system
Examples: Air compressor, flow through nozzles and turbines
Closed system
In a closed system or non-flow system, Energy crosses the system boundary in the form of heat and work, but there is no mass transfer.
Example: Gas contained in the cylinder
Isolated system
In an isolated system no mass and no energy crosses the boundary of the system.
Example: Thermal flask and insulated gas container
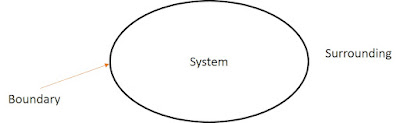
Thermodynamic boundary and surrounding
Boundary
The system and surroundings are separated by the system boundary. It may be real or imaginary.
SurroundingEverything outside of the system which affects the behavior of the system is called surroundings.
Equilibrium
It is defined as state of balance when two forces cancel each other.
Types of equilibrium
Thermal equilibrium
A system is said to be in thermal equilibrium when the temperature at all points in the system is the same.
Example : Isothermal process
Mechanical equilibrium
A system is said to be in mechanical equilibrium when the pressure at all points in the system is the same.
Example : Constant pressure process – Boiling of water
Chemical equilibrium
A system is said to be in chemical equilibrium, if it does not undergo any chemical reaction.
Example : Cool drinks
Thermodynamic equilibrium
A system is said to be in thermodynamic equilibrium when the system available in thermal, chemical and mechanical equilibrium.
Example : Bursting of ballon
Property
The state of the system may be identified or described by certain observable quantities such as volume, temperature, pressure and density etc.
All the quantities which identify the state of the system are called properties.
There are two sorts of property:
Intensive properties
Extensive properties
Intensive and Extensive properties
Intensive (or) Intrinsic properties
These are the properties which are independent of the mass of the system.
Examples: Temperature, pressure, velocity, entropy, density
Extensive (or) Extrinsic properties
These are the properties which are dependent on the mass of the system
Examples: volume, energy, specific volume = volume / mass
Specific enthalpy = Enthalpy / mass
State of the system
It is the condition of the system at any particular moment which can be identified by the statement of its properties, such as pressure, volume, temperature etc.,
Process of the system
It is the change of system state at any particular moment from one equilibrium state to another equilibrium state.
- Change of pressure
- Change of temperature
- Change of energy
Cycle of the system
When a process are performed on a system in such a way that the final state is identical with the initial state. This is known as cycle or cyclic process.
- Otto cycle
- Diesel cycle
- Carnot cycle
- Rankine cycle
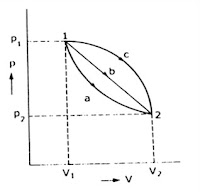
Point and path fuction
Point (or) state function
The quantities which are independent on the process of path followed by the system are known as point functions.
Example : Pressure, volume, temperature, entropy, enthalpy
Path (or) process function
The quantities which are dependent on the process or path followed by the system are known as path functions.
Example : Work done, Heat transfer